What is a battery? Battery maintenance

Battery maintenance
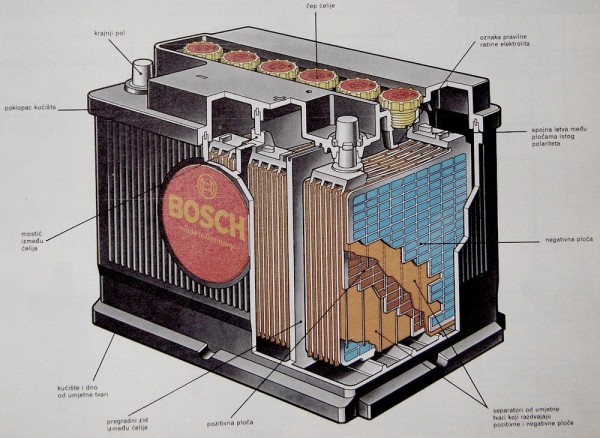
Battery is an energy reservoir that, by converting electricity into chemical (charge), accumulates a certain amount of electricity. By connecting the consumer to its terminals, a reverse process is carried out, that is, converting the chemical into electricity (discharging the battery). It consists of one or more cells containing two types of electrodes (cathode and anode) that are immersed in the electrolyte.
The so-called lead batteries both electrodes are coated with lead sulfate PbSO4. During charging, complex electrochemical reactions occur with the electrolyte (dilute sulfuric acid in a suitable concentration), lead sulfate is converted to lead dioxide (PbO2) at the negative electrode (anode) and pure lead is formed at the positive electrode (cathode). At the same time, the concentration of sulfuric acid (H2SO4) increases. During the discharge, the opposite process takes place, creating a voltage on the electrodes of about 2 V.
The charging and discharging process can be represented by the expression: Battery capacity is expressed in Ah (ampere-hours). In principle, the product of the battery discharge time and the average discharge current should be equal to the battery capacity. However, in reality, the capacity significantly depends on the orderliness of the discharge and charging regime. The battery must not be left without electrolytes, the lead-acid battery must not be discharged below 1,8 V or stand out of use for a long time without recharging, and must not be subjected to excessive electric shocks. The more the battery is discharged or charged with a higher current, the smaller the capacity will be (so it will maintain sufficient voltage for a shorter time). Battery capacity also decreases at low temperatures, which is why battery problems are more common on vehicles in the winter months.
Direct short circuits at battery terminals (especially high-capacity ones) can be very dangerous, as they produce real fireworks of strong sparks that can cause severe burns, fire, vision damage, and even lead to explosive battery breakdown.
In normal operation, the battery gradually loses its electrolyte water. Therefore, the electrolyte level, which must cover the battery panels by approximately 10 mm, should be checked periodically. Only (preferably distilled) water should be added if necessary, not acid, as the acid is not lost by evaporation. Water loss is reduced for batteries declared as maintenance-free, but after a long time, these should be checked.
Charging the battery
The battery is charged on the vehicle by providing an alternator with a constant voltage of 14V. It's a good system, but it doesn't apply with battery chargers because it would take a long time for the system to fully charge the battery. Three or more charging systems are used with battery chargers depending on the type and quality of the charger:
1. constant current
2. constant voltage
3. combined
For charging with constant current, a current of 1/10 capacity for 12 hours is used. It should be noted that charging takes 12 instead of 10 hours which means that lead batteries have 20% loss when charging. The voltage rises from 1,8 to 2,7V per cell. This is the fastest method of normal charging and has the disadvantage that at the end of charging there is an intense knocking in the cells which is a consequence of electrolysis of water where as a product a mixture of hydrogen and oxygen which is very explosive occurs. Also, the evaporation product is a denser acid residue, so when the electrolyte level in the battery decreases (it should be about 10 mm above the plates), only distilled water should be added.
With constant voltage charging, there is no strong evaporation because it charges 2,4V per cell, but there are drawbacks because at the start of charging, when the battery is empty, it draws large currents, which is harmful to the battery. Charging is complete when, at a voltage of 2,4V per cell, the current drops to 1/100 of the capacity.
Combination charging is best and is used whenever possible. The charging procedure is as follows. First, the charging starts at a constant current of 1/5 capacity and then charges until the voltage per cell reaches 2,4V and then switches to charging at a constant voltage until the end. Charging is complete when, at a voltage of 2,4V per cell, the current drops to 1/100 of the capacity.
Important Note: The battery must not be plugged in or unplugged while the AC adapter is on. This is because when the crocodile clips are connected, when the power adapter is turned on, sparking can occur and may explode.
Insufficient filling
Insufficient charging occurs when the battery does not receive enough charge to return to a state of full charge; this will lead to slow sulfation. This error can occur when the vehicle is rarely used for short trips or when used for stop-start city driving. Insufficient charging also occurs if the alternator voltage is below 13,6 - 13,8 V.
Battery maintenance
How to save your battery
1) Maintain electrolyte levels. If your battery has plugs that are removable, you need to open them and check the fluid level in the cells, and add clean distilled water as needed. NEVER add new or used sulfuric acid, it will only cause your battery to fail before it goes down. In order to prevent a battery defect, it is necessary to check whether the electrolyte level is approximately 10 mm above the upper edge of the separator, if it is lower, to add exclusively distilled water.
2) Keep terminals clean. Visually inspect terminals and cables at least once a year, especially at high outside temperatures, for corrosion. If corrosion occurs, clean it with a scraper and a steel brush. The terminals must be properly cleaned and tightened, reducing current resistance when starting the engine, and saving battery.
3) Keep the battery housing clean. Do not allow dust, residues from engine oil and other impurities to remain on the battery housing. It should always be clean and dry.
4) Keep the battery charged. If you have not used your vehicle for weeks or months, recharge the battery before reusing. The lack of use of the vehicle leads to the self-discharge of the battery, the formation of sulfate deposits, and consequently the deterioration of the battery. The charging method and time depends on the type of your battery. Therefore, it is important to keep your battery charged.
5) Keep the electrical installation in good working order. The correct condition of all electrical components in the vehicle extends the life of your battery!
Battery failures
In addition to external mechanical damage (melted stairs due to poor contact on terminals, which can be re-poured and cracking of the box for which there is no cure), there are also internal failures such as:
1) Sulphation occurs when the battery is discharged. It has already been said that discharging the battery below 10,8 V is not allowed as permanent damage occurs (dragging the soluble sulfate over the plates). This type of failure is recognized by the greatly reduced battery capacity. This malfunction can be partially remedied by charging the battery with electricity of 2% capacity for 48 hours.
2) A short circuit occurs due to a failure in the manufacture of the battery. Somehow the separator is damaged and there is a short circuit between the + and - plate. This type of failure can be determined by measuring the density of the electrolyte. The density of electrolytes in all cells must be uniform. Different densities certainly indicate a short circuit. Different densities in cells can also occur due to internal cracks in the partitions between cells. Since the cells are at different voltage levels, that cell is discharged. Neither of these two cases can be fixed.
3) Interruption, this type of malfunction is caused by poor fabrication in the factory. When assembling a battery when cells are inserted into a box, the connections between the cells are made by electrically resistant welding. Failure to perform this procedure at a good time will result in interruption at that point. The interruption can be observed when the instrument measures uncharged battery voltage is good and when we turn on some consumer voltage drops to almost zero. The assumption that the battery is interrupted can be confirmed by measuring the electrolyte voltage between adjacent cells. First we connect the battery to the consumer, the voltage at the poles is significantly reduced then we measure the voltage between adjacent cells in sequence and the voltage must be about 2V however between the cells where the break occurred the voltage is much higher and the reverse polarity.
Why batteries get lost too soon
In the US, battery replacement is a $ 17 trillion deal. A total of 75% of these "starter", "strong", "auxiliary", "industrial", "marine" and all other batteries suffer from the formation of stiff sulfate deposits on lead plates, while 25% of the batteries suffer from mechanical damage.
The first, and most common cause of battery damage, is the formation of abnormal amounts of hardened sulfate deposits on the lead plates. Sulfuric acid cannot enter the pores of the lead plates, and the battery begins to die. The process of sulfation is a normal occurrence in lead batteries, so during the discharge phase some crystals remain on the lead plates, until the normal filling returns them to the electrolyte. The other 25% of the batteries are damaged due to mechanical damage (short circuit, or separation of the connection between the plates, etc. ().
By plate sulphatization we mean the formation of large crystalline lead sulphate, which is captured in the form of thick bark on the surface of the plates. Its presence prevents the chemical processes from taking place properly in the battery itself. The consequence is a decrease in capacity, and if the sulfatization is more extended the battery is more unusable.
The causes of sulfatization are:
- discharging the battery below the allowable voltage limit, ie. below 1.83 V, so it is left empty for a long time,
- deep discharge,
- improper charging and discharging, especially in terms of current,
-filling with sulfuric acid instead of distilled water or excessive electrolyte concentration,
- self-discharge, because the lead-acid battery discharges itself if it does not work for a long time,
- discharge the battery under high temperature, especially in the event of a short circuit.
The signs of sulfatization are:
- the surface of the plates acquires a lighter color, changes from lead gray to whitish on the negative plate, and the positive dark brown changes to light brown,
- the surfaces of the slabs are rough, when you touch it with your finger you can feel fine sand (sulphate crystals),
- if a knife is pulled over the surface of the negative plate, no bright metal trace remains,
- the internal resistance of the battery increases so that when charging such a battery the voltage increases up to 3.0 - 3.5 V (UA = EMSa + 1 • Ru)> and when discharging the voltage is below normal
- electrolyte concentration is reduced, as well as capacity.
The elimination of sulfation is the conversion of lead sulfate back into sponge lead, ie. lead oxide and sulfuric acid.
Ratio of acid density and battery charge:
| charge | acid | stress | Freezing temperature |
| 0% | 1,05 | 11,80 V | - 7,7 ° C |
| 25% | 1,12 | 11,90 V | - 10,8 ° C |
| 50% | 1,16 | 12,10 V | - 17,9 ° C |
| 75% | 1,21 | 12,36 V | - 31,7 ° C |
| 100% | 1,26 | 12,60 V | - 56,5 ° C |
Degree of battery discharge - in case the density in all cells is approximately the same (density deviation up to a maximum of 0,003 g / cm3) - according to the following, approximate classification: http://inverter.eu5.org/akumulator.htm
1,240 to 1,290 g / cm3 = full battery
1,200 to 1,240 g / cm3 = partially discharged battery
below 1,200 g / cm3 = empty battery
- sulphated battery - in case the density of electrolyte in all cells is approximately the same, but very low (<1,140 g / cm3 and less) and the battery has not been electrically charged for a long time.
- poor sealing between cells - in case the density of two adjacent cells is less than 0,03 g / cm3 by more than in other cells (warning: possible replacement with short circuit signs!)
- cell short circuit - in case the cell density (which is in short circuit) is significantly lower than the electrolyte density in other cells (by> 0,03 g / cm3)
- battery overcharge - when the density in all cells is greater than 1,300 g / cm3
Buying a battery
Before buying a new battery: check if the old battery is worn out and if a replacement is really needed or if the cause of wear or discharge is a fault in the vehicle. Faults such as a hidden power consumer on the vehicle or insufficient alternator charging voltage often occur. When you have determined that the battery needs to be replaced, then it is your turn to choose.
When selecting, it is important to check the battery power (eg: 55 AH). Then, depending on which vehicle the battery is fitted in, it is important to pay attention to quality. Because there are a large number of consumers on modern cars and it is important that the battery also has the necessary reserve of power.
Special attention should also be paid to the conditions in which the car is used (eg if these are short distances with frequent ignition, or not using the vehicle for several days…)
The selection criteria are:
- conditions of use of the vehicle,
- vehicle type (by consumer number),
- warranty period (usually 2-3 years),
- Dinner
After purchasing the battery, it is a good idea to read the warranty card, which describes how to use the battery, in order to avoid problems with complaints and to get basic information about the course of the complaint procedure.
The average battery life is about 3-5 years, but with the right choice and proper use, it can be extended to 6-7 or more years.
Checking the fluid level in the battery
An electrolyte, that is, a battery fluid, is a mixture of sulfuric acid and distilled water. The required electrolyte level in the battery is just above the battery panels, which are visible through the charging holes at the top. Some batteries are sealed and do not need replenishment, but in many cases fluid levels drop over time due to evaporation of electrolyte water.
To check and replenish the battery, remove the plugs or cover from the charging port and top up to the required level with distilled water. Attention Only add distilled water to the battery and nothing else! Some batteries have a transparent housing with an external label of the required fluid level.
Caution is required when handling electrolyte fluid as it is corrosive and can damage your skin and vehicle color. If you come into contact with a liquid, be sure to rinse it with a strong stream of running water.
Before installing plugs or covers on the battery, make sure that the ventilation holes on them are clean. If they are clogged, clean them with a toothpick or a piece of thin wire.
Leti tj. at higher outside temperatures, check the liquid level in the battery more often, as evaporation is stronger. If the fluid level in one cell drops more than in others, it is probably damaged and you may need to replace the entire battery. The cells are interconnected, so if one is damaged, the battery will not be able to be charged. If you suddenly need to refill a larger amount of liquid, the battery may charge more than necessary. Have your car electrician check the power connections.
Checking the battery connections
There are several types of battery connectors, but the same applies to all. The connecting surfaces of the connectors and the battery terminals must be clean of dirt and corrosion and tightened tightly. Use vinegar to clean the dirt, then clean the terminals with sandpaper or wire brush until the metal is shiny. Clean the connector faces with sandpaper or a small fine file. Lubricate the terminals with oil gel and reattach the connectors.
Charging the battery
Frequent shorter trips at night or in bad weather cause more energy to be consumed by the batteries for powering the headlights, wipers, heating than the engine generator can replace. The battery will be too weak to start the starter. An early sign of a weakening is when the engine barely turns at ignition. Check the joints for proper connections, remove the battery and take it to a mechanic's workshop or charge it yourself. You can find different types of chargers in our offer. Buy one that has a high and low charge and an ammeter that shows the charge level while it is running. Before charging, remove the battery cell plugs or cover to allow gas to escape when charged with electrolytic fluid. Charge the battery in an open and ventilated area, otherwise the spark can ignite the gas that evaporates from the battery.
Most batteries have a capacity of 48 amps which means it can deliver 48 amps per hour, 4 amps per 12 hours, etc. Therefore, a charge of 2 amps takes 24 hours to charge a completely discharged battery. In an emergency, charging from a high 10 amps, 30-60 minutes of which is enough to start the car. However, frequent heavy charging can damage the battery - it is better to charge with less power for more hours or even leave overnight.
According to the manufacturer's instructions, set the charger to high or low power, as needed. Unplug the battery wires, if possible, remove the battery from the car. Connect the crocodile clips on the charger black wire to the negative (-) terminal and the red wire to the positive (+) terminal. Plug the charger into an outlet and plug it in. Some chargers automatically shut off when the battery is fully charged, which is certainly a convenience. Otherwise, you must evaluate by elapsed time and by checking the electrolytic fluid with a battery hydrometer. In both cases, always unplug the charger before removing the clips and reconnecting the battery wires.
Ignition cables
If you run out of battery, a workaround might be to disconnect the battery from another vehicle using cables. You will connect the cables by connecting the positive terminal of the car from which you are taking power to the positive terminal of your vehicle. You should do the same with negative genders. Before attempting to start an engine with bridged cables, it is important that the car from which you are taking the power is on.
Battery replacement
To remove the battery from the car, you must first remove the terminals, first the negative, then the positive pole. When you put a new battery in the tray, replace the terminals in the reverse order, first positive, then negative.
Finally, if you have trouble starting the engine, always wait for the battery to "rest" for a while, as it will be easier to discharge in these situations.
Sources:
auto-mane.com
inverter.eu5.org
Recommendation of similar texts:

Hi there, I am Mladen and I am an auto enthusiast. I started this blog years ago to help like minded people share information about latest cars, car servicing ideas, used car info, exotic cars, and auto technology. You will find helpful articles and videos on a wide variety of cars - Audi, Mercedes, Toyota, Porsche, Volvo, BMW and much more. Ping us if you have anything cool to share on latest cars or on how to make older cars more efficient, or just want to say hi!









Greeting new member of
I was interested in the density of the electrolytes at about + 15 ° outside temperature, I measured the bometer and in two cells it was about 1250, about 1260 remained. Charging 5a and 14,5v the charger came to 7v and 8a at 16-0,5 hours at one time it stopped charging. After about an hour, I examined the electrolyte and the condition did not improve significantly. I left it that way and after a couple of hours put it back into charging mode 1a and 16v after say an hour the bubbles started to burst and it was still in that mode. The electrolyte is clear all cells were filled normally. The pre-fill state was 12.36 and 67% full. It's a 75ah 690EN nameplate battery. It stands in a car that drives a few miles during the day. What I was most interested in was whether the electrolyte density could be equalized in all cells and be about 1268g / cm3
Thank you